| |
THERAPEUTIC STRATEGIES TO IMPROVE BRAIN FUNCTION |
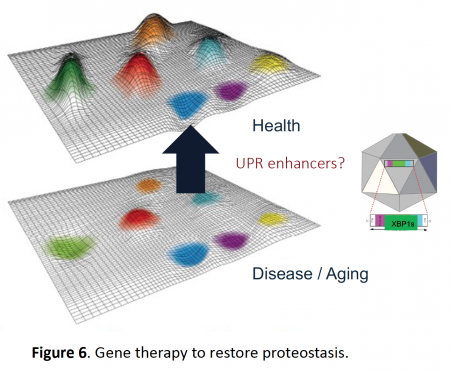
ER stress is a salient attribute of many human diseases including obesity, liver disorders, cancer, diabetes and neurodegeneration (Figure 5) (Hetz et al., 2020 Nature Rev Mol Cell Biol). The pathways that determine the decay of brain function as we age and in disease are not well understood. Thus, it is necessary to evaluate new concepts and identify novel targets. Because the decay in the buffering capacity of the proteostasis network is a central pillar of the aging process, and XBP1 and ATF6 are master regulators of the UPR, we hypothesize that the artificial activation of the UPR may delay normal brain aging and protect against neurodegeneration by (i) strengthening neuronal proteostasis, (ii) reducing protein aggregation and (iii) improving synaptic function. We have developed new technologies using gene therapy and adeno-associated viruses to reprogram gene expression and enforce adaptive proteostasis repair pathways (Figure 6), with demonstrated success in multiple models of neurodegeneration including Alzheimer´s disease (Duran et al., 2023 Mol Ther), Parkinson and Huntington´s disease (Vidal et al., 2020 Mol Ther; Valdes et al., 2013 PNAS), mechanical injury to the CNS (Valenzuela et al., 2012 Cell Death Diff; Oñate et al. 2019 Cell Death Diff), in addition to normal brain aging (Cabral et al., 2023 EMBO J). We have generated several patent applications and licensed three UPR-based technologies to UCB in Belgium.
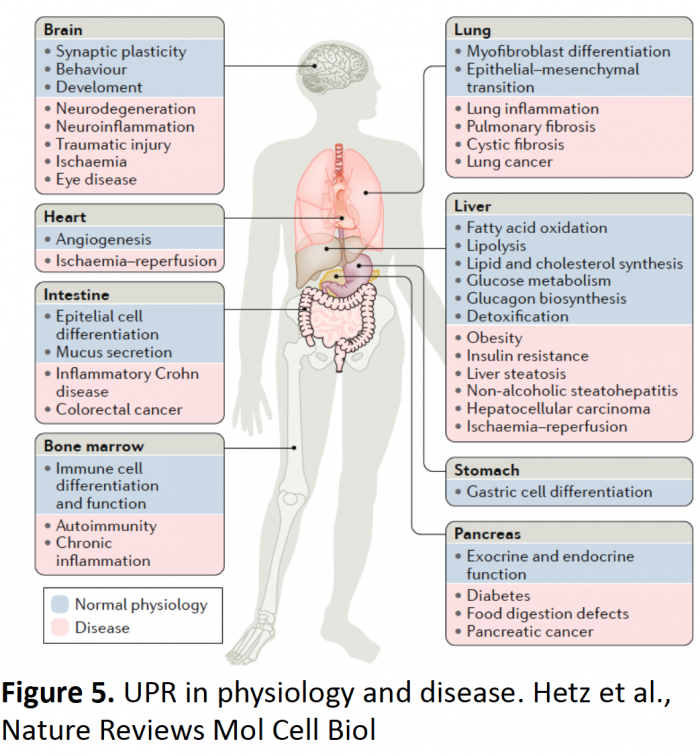
ER stress is a salient attribute of many human diseases including obesity, liver disorders, cancer, diabetes and neurodegeneration (Figure 5) (Hetz et al., 2020 Nature Rev Mol Cell Biol). The pathways that determine the decay of brain function as we age and in disease are not well understood. Thus, it is necessary to evaluate new concepts and identify novel targets. Because the decay in the buffering capacity of the proteostasis network is a central pillar of the aging process, and XBP1 and ATF6 are master regulators of the UPR, we hypothesize that the artificial activation of the UPR may delay normal brain aging and protect against neurodegeneration by (i) strengthening neuronal proteostasis, (ii) reducing protein aggregation and (iii) improving synaptic function. We have developed new technologies using gene therapy and adeno-associated viruses to reprogram gene expression and enforce adaptive proteostasis repair pathways (Figure 6), with demonstrated success in multiple models of neurodegeneration including Alzheimer´s disease (Duran et al., 2023 Mol Ther), Parkinson and Huntington´s disease (Vidal et al., 2020 Mol Ther; Valdes et al., 2013 PNAS), mechanical injury to the CNS (Valenzuela et al., 2012 Cell Death Diff; Oñate et al. 2019 Cell Death Diff), in addition to normal brain aging (Cabral et al., 2023 EMBO J). We have generated several patent applications and licensed three UPR-based technologies to UCB in Belgium.