| |
ORGANELLE STRESS SIGNALING |
MISSION: This laboratory focuses on understanding the molecular programs underlying the adaptation of cells to perturbations on organelle function, its relationship to pathological conditions affecting the nervous system, and the development of prototypic therapies to prevent this damage.
Proteostasis networks and ER stress – Alterations in protein homeostasis (referred to as proteostasis) have devastating consequences for the proper function of the cell. Stress injuries initiate multiple signaling responses, either to adapt to the new condition or to activate specific apoptosis pathways if a critical threshold of damage has been reached. Our laboratory is committed to the study cellular strategies involved in adaptation to chronic organelle damage, which are linked to several brain diseases. The endoplasmic reticulum (ER) has important functions to sustain proteostasis, highlighting its role as a sophisticated machinery for folding, quality control, and secretion of proteins. Alterations on ER function lead to the accumulation of unfolded proteins at its lumen, a cellular condition termed “ER stress”. ER stress engages an integrated signaling pathway known as the “Unfolded Protein Response” (UPR), which aims to restore homeostasis. Nevertheless, the mechanisms that control the transition from an adaptive state to cell death programs remain poorly understood and are a central subject of our research.

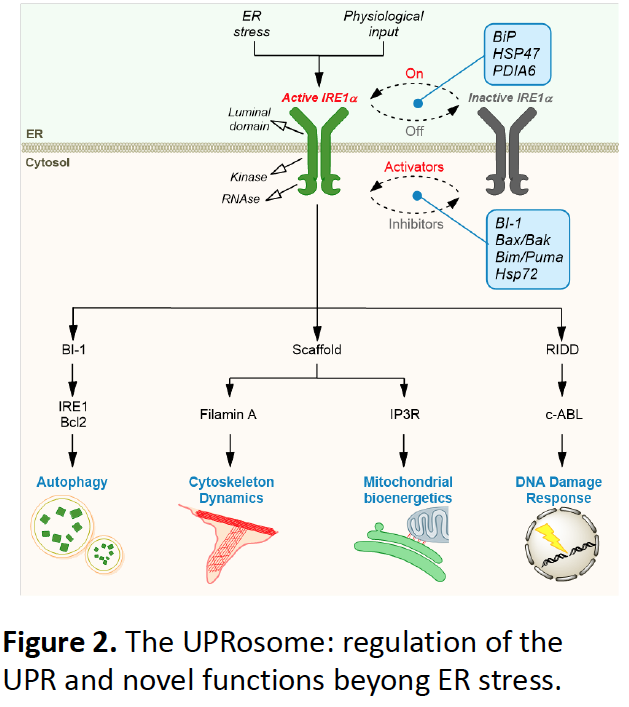
The UPRosome
Non-canonical roles of the UPR
Recent advances in the field have demonstrated components have multiple functions in biological processes that are beyond maintenance of ER proteostasis. The identification of novel binding partners of UPR stress sensors has provided mechanistic insights, indicating that the formation of distinct protein complexes serves as a platform for interorganellar communication and signaling crosstalk to regulate mitochondrial bioenergetics (Carreras et al 2019 Nature Cell Biology), cytoskeleton dynamics (Urra et al., 2018) and DNA damage (Difei et al 2020 Nature Com). We interactome screenings and unbiased approaches followed by molecular cellular and in vivo validation to assess these novel functions. We enforce integrative approaches to address this function, combining multiple animal models (flies, zebrafish and mice), cell biology studies and biochemistry.
Regulation of apoptosis and lysosomal function
At the ER membrane, a variety of stress signals are integrated toward determining cell fate, involving a complex cross talk between key homeostatic pathways. In this context, key regulators of cell death of the BCL-2 and TMBIM/BI-1 family of proteins have relevant functions as stress rheostats mediated by the formation of distinct protein complexes that regulate the switch between adaptive and proapoptotic phases under stress. Our group has contributed to understand how cell death under ER stress is regulated, identified new regulators of apoptosis (Rojas et al 2012 Cell Death Diff), lysosomal permeabilization (Pihan et al 2020 Science Advances) and the relationship between the apoptosis machinery and UPR signaling (Lisbona et al., 2009 Mol Cell; Rodriguez et al., 2012 EMBO J).